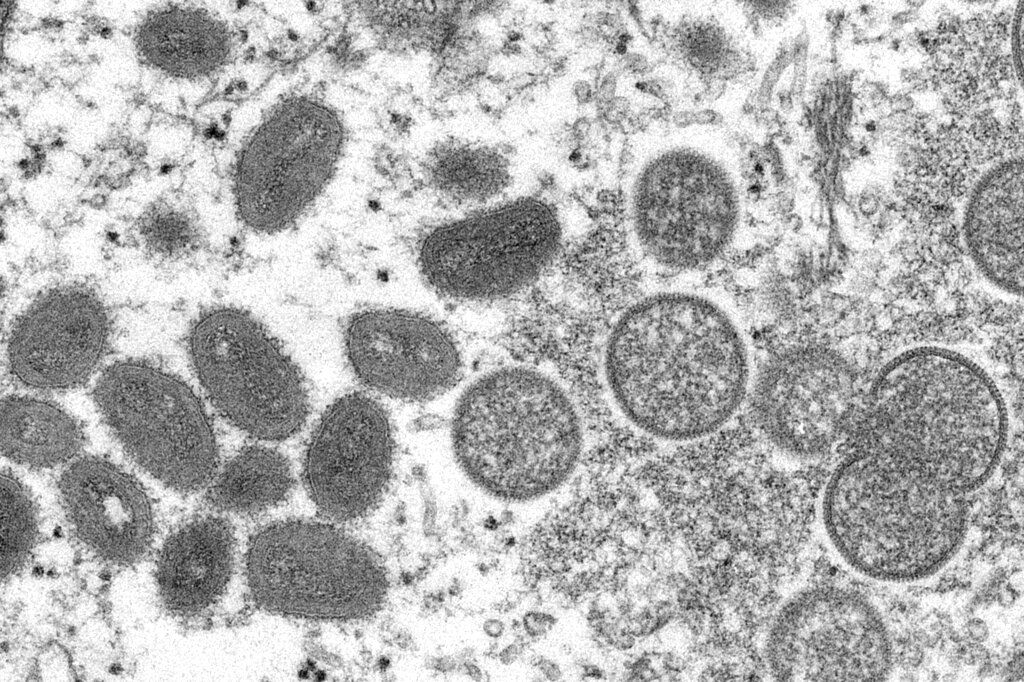
With monkeypox cases slowly but steadily rising across the world, diagnostic companies are rushing to develop tests for the disease in hopes of tapping into a nascent market as governments around the world try to trace the first major outbreak of the disease outside central Africa.
Much like early 2020 when diagnostic companies jumped in on efforts to detect and contain the COVID-19 pandemic, the rush for monkeypox tests began last month after a case was detected in Massachusetts in the US.
Also read | WHO explains how to contain monkeypox
Despite the similarities, however, the monkeypox market is unlikely to be the multi-billion dollar boon that the COVD-19 test market was for companies: there already exist vaccines, treatments, and tests for monkeypox, all of which were missing in the case of COVID-19 when it was first detected.
Further, monkeypox, unlike COVID-19, poses a far lesser public health risk as it is not as easily transmissible and far less lethal: monkeypox spreads through close contact and can cause flu-like symptoms in addition to pus-filled lesions on the skin. However, these symptoms typically subside on their own in weeks.
Also read | UAE becomes first Gulf country to report monkeypox case
Yet, with around 550 cases detected in 30 countries around the world, several governments in Europe are looking to pre-emptively ramp up testing capacities.
Concern about the extent of potential infections has also been exacerbated by the World Health Organization’s (WHO) warning that the number of infections in Europe could rise through the summer when people gather for various festivals and parties.
Also read | Does monkeypox need strict quarantine measures yet? Here’s what US President Joe Biden said
In light of said warning, countries in Europe are ramping up capacities. While the Netherlands and Switzerland, where only a handful of monkeypox cases have been reported, have said that they have sufficient testing capacity, other countries like the UK, where around 200 cases have been reported, are expanding capacity.
It is this growing concern that is driving pharmaceutical giants like Roche to manufacture test kits for monkeypox. While said kits have not yet received regulatory approval for emergency use, they are being used for research purposes.
Also read | What experts are saying about the monkeypox outbreak
In a similar vein, several listed Chinese pharma giants, including Jiangsu Bioperfectus Technologies, have started to manufacture monkeypox test kits with the European Union’s (EU) CE mark of quality to enable their sale in Europe.
That being said, analysts suggest that the monkeypox market is unlikely to become as big as the COVID-19 testing market, and some say that this is an attempt by big pharma to try and offset the decreasing demand for COVID-19 tests, which had contributed to billions of dollars in earnings over the past couple of years.
Also read | Netherlands directs 3 weeks of quarantine after contact with monkeypox patients
“This isn’t going to be the next COVID … so I don’t think the needs are massive. I don’t anticipate [test] supply to be an issue,” Daniel Bausch, the senior director of emerging threats and global health security at global diagnostics alliance FIND, was quoted as saying by Reuters.
“It would be very difficult for monkeypox revenues to offset this in any meaningful way,” Barclays analyst Emily Field also told Reuters.