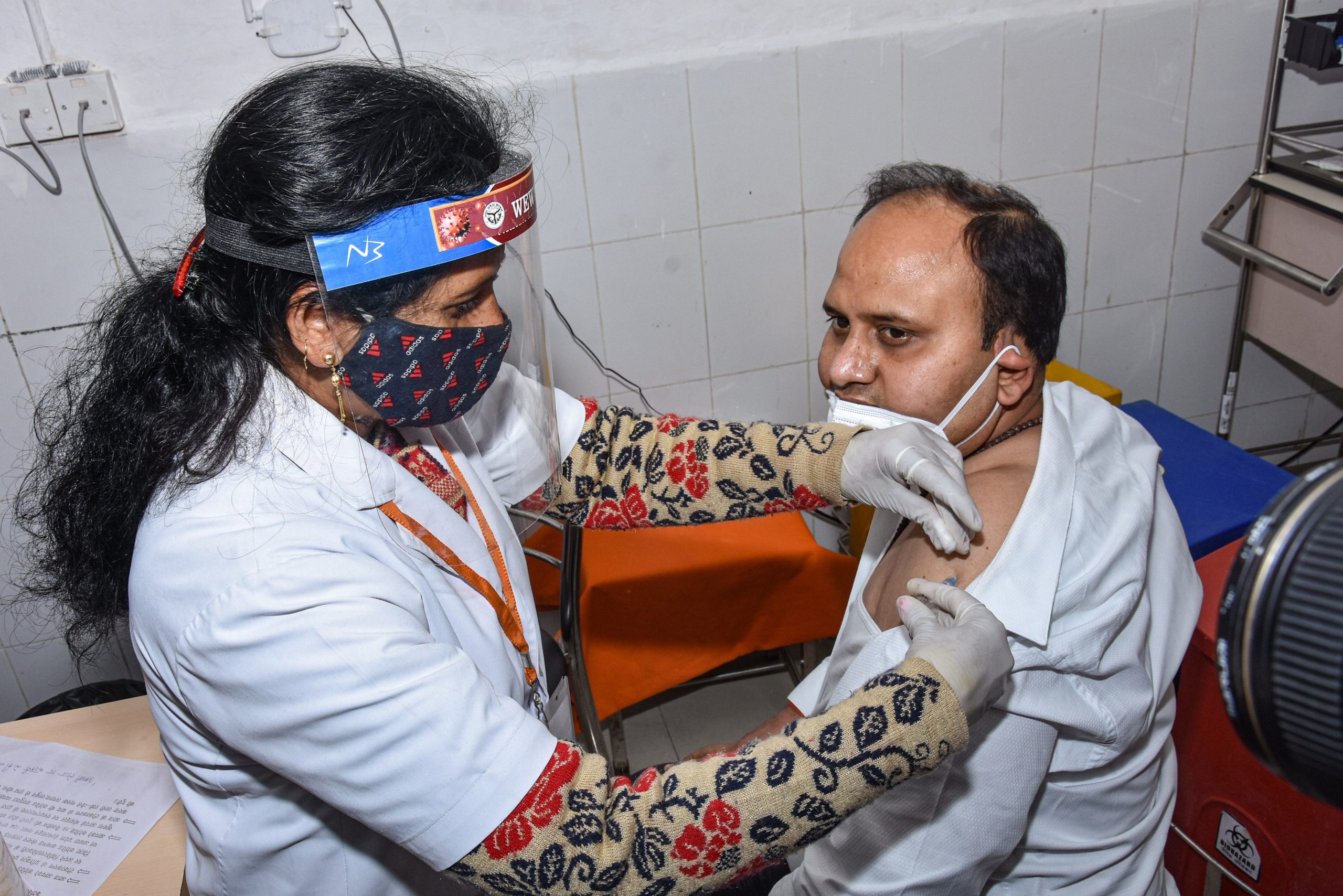
A day after India started its vaccination programme, the Health Ministry said that a total of 447 adverse events following immunisation have been reported so far, of which only three cases required hospitalisation, reports PTI. The ministry said that most of the adverse events that were reported included fever, headache, and nausea.
According to the ministry,a total of 2,24,301 beneficiaries have been administered COVID-19 vaccine since nationwide drive was rolled out on Saturday.
On day 2 of the drive, only 17,072 beneficiaries were inoculated in six states. Health Ministry joint secretary Manohar Agnani said it is a usual strategy to avoid days on which there is a holiday or routine immunisation for other diseases. “Most states are doing it over four days but smaller states like Goa are doing it over two because they have lesser numbers,” he added.
Injecting confidence in the people, several high profile persons, including AIIMS director Randeep Guleria, NITI Aayog member VK Paul, who is also head of an empowered group on medical equipment and management plan to tackle the coronavirus outbreak, Serum Institute of India (SII) CEO Adar Poonawallah, West Bengal minister Nirmal Maji, BJP MP Mahesh Sharma, who is a doctor by profession, Chairman, Apollo Hospitals Group, Dr Prathap C Reddy and Chairman of Manipal Hospitals Sudarshan Ballal also received their first shot of the two-dose vaccine.
India approved two vaccines, one developed by Oxford University in collaboration with pharmaceutical giant AstraZeneca, being manufactured by SII, and other indigenously developed vaccine by Bharat Biotech on January 1.