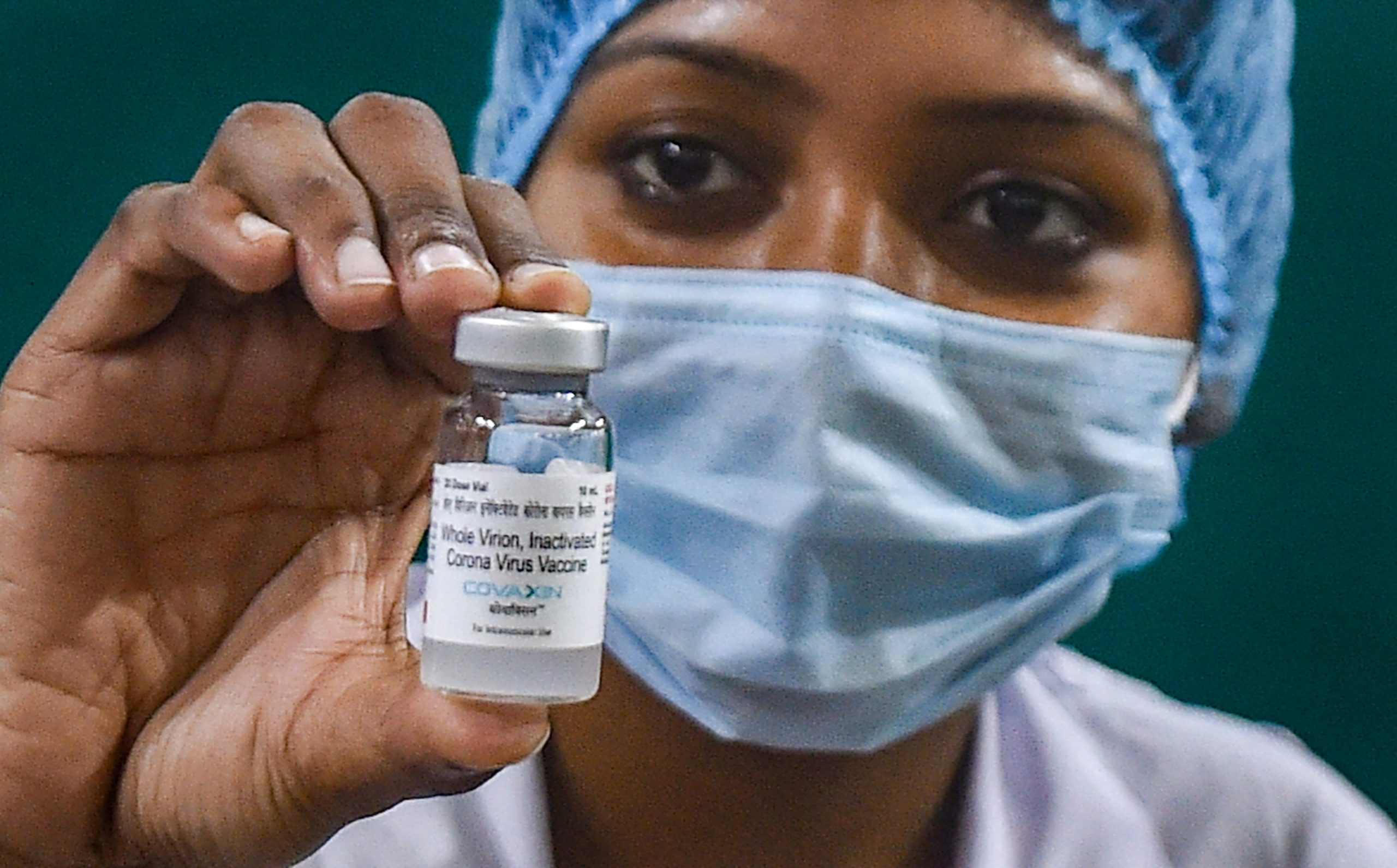
After Chhattisgarh’s Health Minister wrote a letter to Union Health Minister Dr Harsh Vardhan over safety concerns regarding India’s indigenous coronavirus vaccine, COVAXIN, the latter responded by telling the former to address vaccine hesitancy. Chhattisgarh Health Minister T S Singh Deo had requested Vardhan to “halt the supply of COVAXIN” to the state until safety issues were addressed.
Also read: Bharat Biotech issues fact sheet on COVID-19 vaccine amid safety concerns
Among the safety concerns he mentioned were “the inhibitions regarding the incomplete 3rd phase trials of COVAXIN” and “the absence of expiration date on the vials of the vaccine.”
In his response, the Union Health Minister accused Deo of stoking inhibitions regarding the efficacy of the vaccine.
“In such unprecedented times, you should help address any vaccine hesitancy & do what’s in best interest of people, not further vested interests !” he said in his response.
He said COVAXIN was granted the emergency use approval “after completing due diligence & following protocols establishing their safety & immunogenicity.”
Addressing Deo’s concern over the absence of expiration date on the vials, Vardhan shared pictures of vials with expiry date written on it.
He further noted that despite ample supplies of the vaccine in the state, only 9.55% of frontline workers received the first dose of the vaccine.
“Rather than sensationalising non-issues, kindly focus on improving vaccine coverage in the State,” the Union Health Minister tweeted.
Hyderabad-based Bharat Biotech’s COVAXIN was granted approval for emergency use on January 3, despite the lack of data of phase III clinical trials. AstraZeneca’s COVID-19 vaccine, manufactured at Pune’s Serum Institute is the other vaccine that is in use in India.
The country started its mass vaccination drive on January 16 and 7,017,114 people have received the first dose of either of the two vaccines.